‘Further information on this topic can be found in the commercially independent site swimming@home’
Why is pH so important? Keeping a balanced pH is necessary for a safe swim and will extend the life of your swimming pool.
1. The pH value affects the amount of hypoclorous acid (free available chlorine) that is formed, and therefore determines the effectiveness of the chlorine as a killer of bugs.
- At pH 6.5, 90% of the chlorine will be hypochlorous acid
- At pH 7.5, 50% of the chlorine will be hypochlorous acid
- At pH 8.0, 20% of the chlorine will be hypochlorous acid
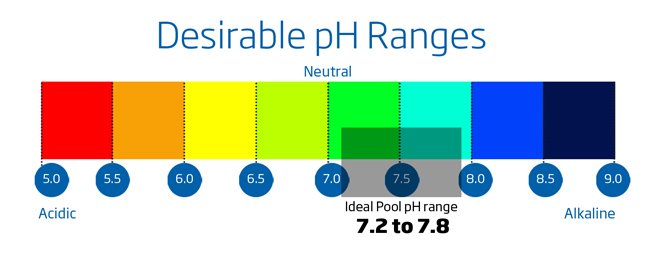
- Unfortunately you cannot run your pool at pH 6.5 – it would acidic enough to corrode the metal fittings in your pool circulation system and it is too far from the human body’s pH of 7.4 to be comfortable to bathe in. The compromise is 7.2 to 7.6, preferably midpoint of 7.4. Remember, if you let the pH drift out of this range, you will have to use more chlorine to get adequate disinfection.
2. Bather comfort. At high pH, the water will make your eyes sting and possibly give you a sore throat
3. At high pH there are two dangers. (1) The danger of scale forming on your pool surfaces, pipework and fittings. This is because at a pH of around 8.0, the calcium in the water combines with carbonates in the water. Result? Calcium carbonate or scale. (2) Calcium carbonate can form into tiny particles and float around in the water giving it a cloudy, turbid appearance.
4. A low pH can corrode metals, eating away at copper fittings and heat exchangers leaving metal oxides to stain pool surfaces. Under certain conditions the precipitated (particulate) metals can tint your hair, giving you a rather dated appearance in these post-punk times!